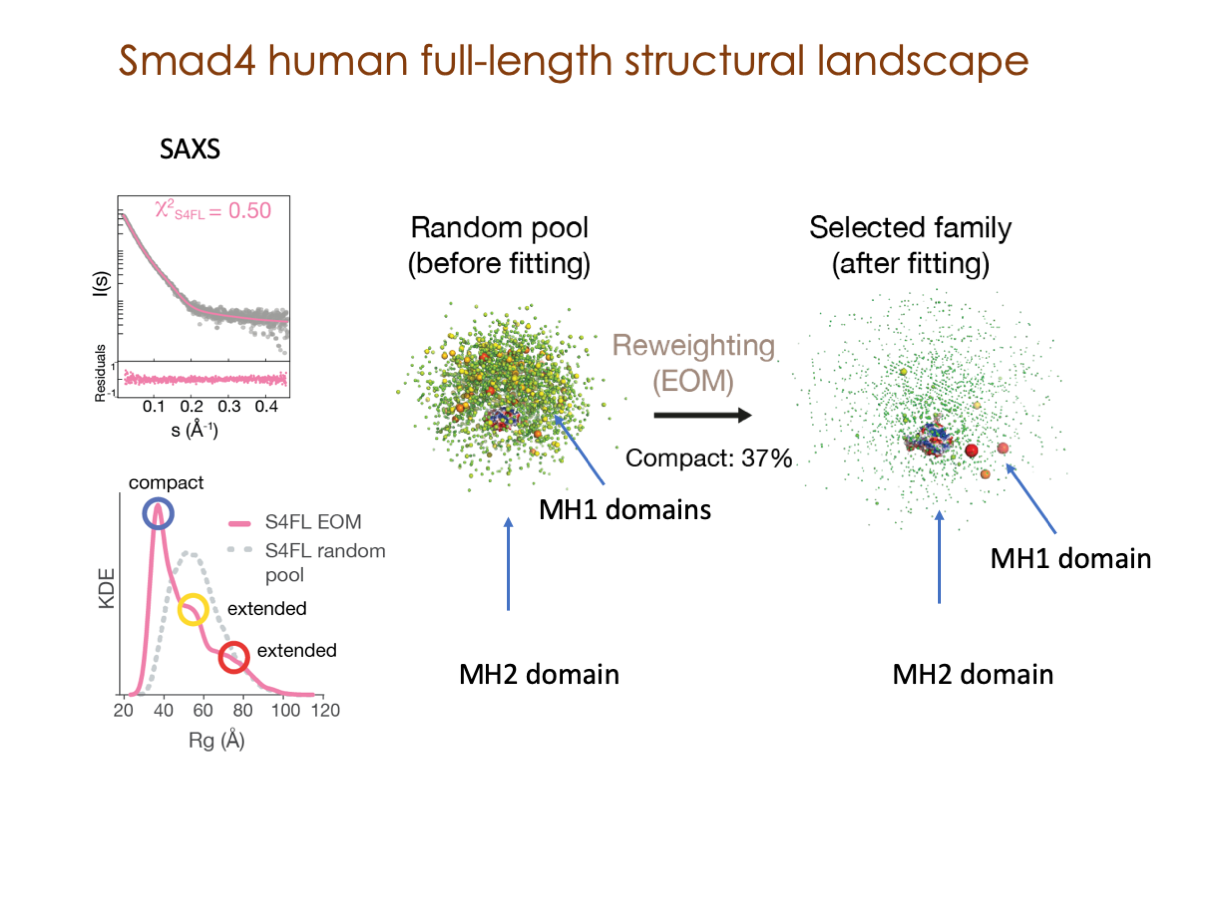
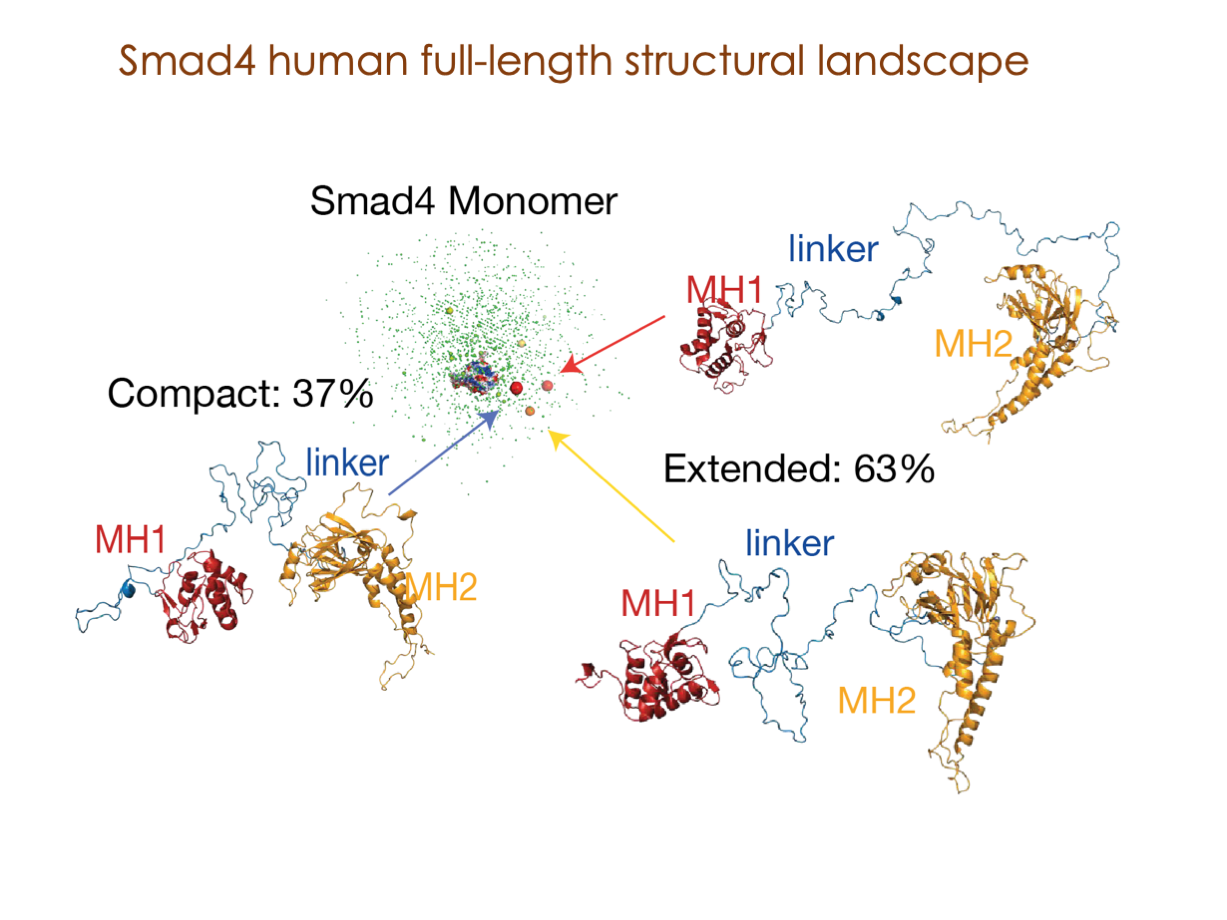
Our primary research interests are centered on deciphering intricate mechanisms that bridge cell signaling and gene expression, comprehending the regulation of these essential processes, and exploring their profound implications in a spectrum of human diseases.
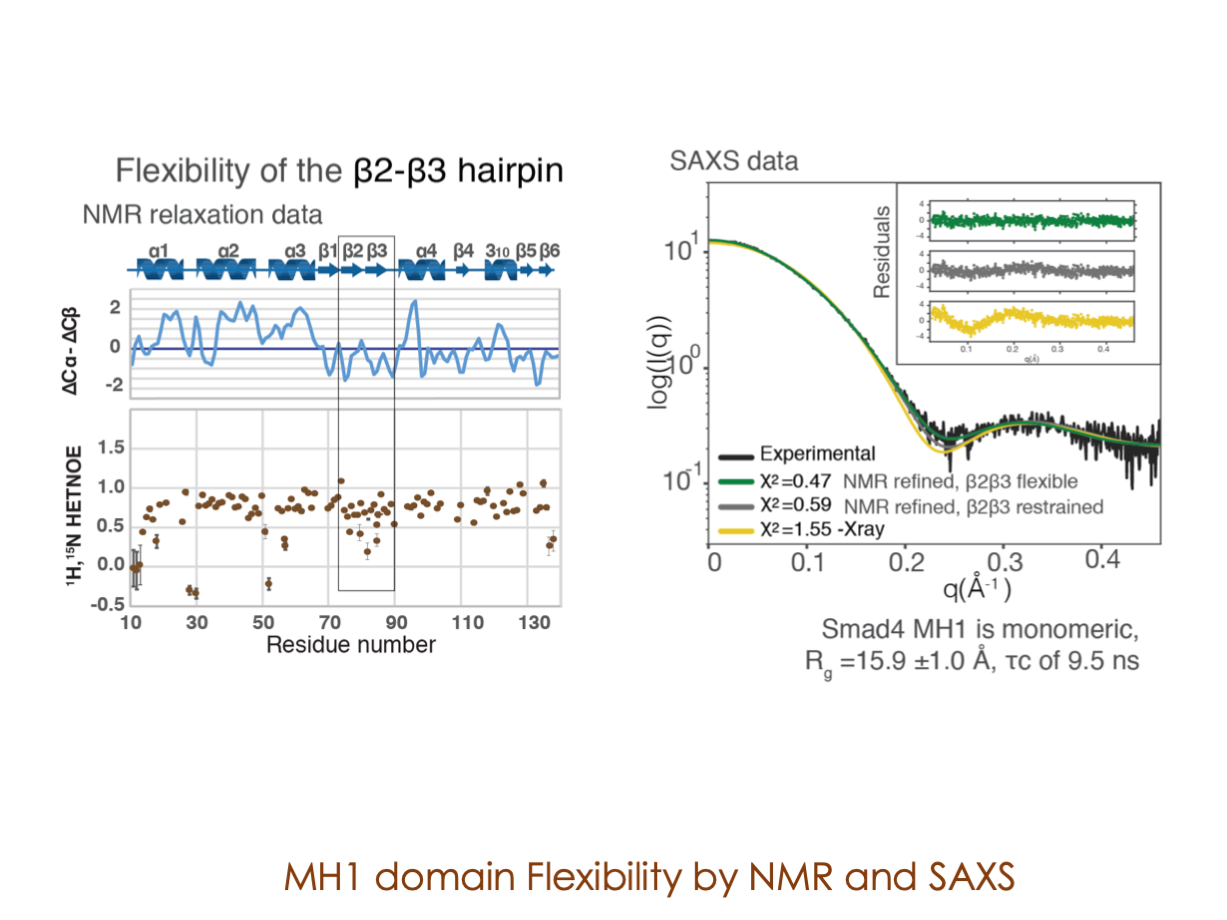
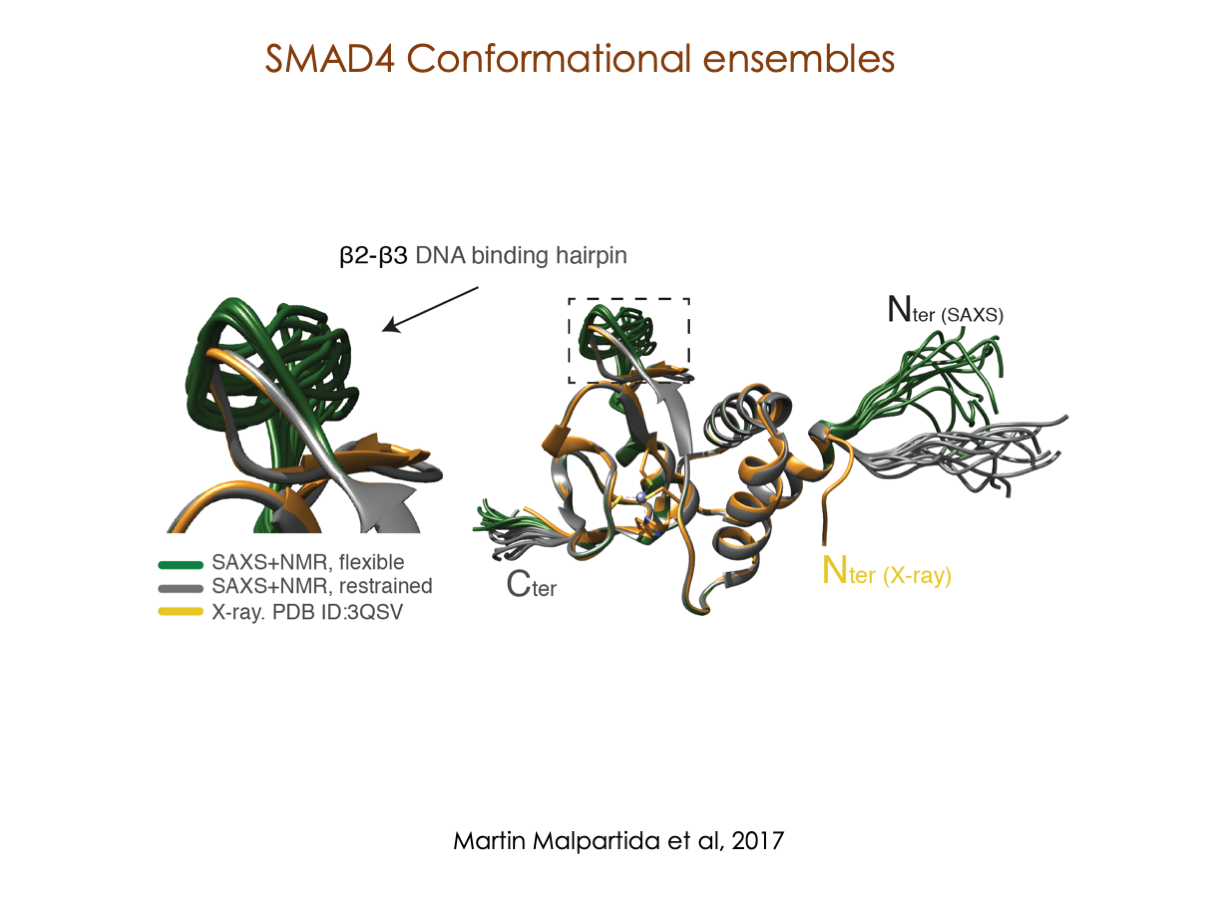
In our laboratory, we seamlessly integrate cutting-edge molecular biology techniques with state-of-the-art tools, including NMR, X-ray, and SAXS, alongside the power of bioinformatics. These methods illuminate the structural intricacies of these molecular complexes. Furthermore, we have incorporated cryoEM into our array of structural biology techniques to delve into the study of expansive and multifaceted systems.
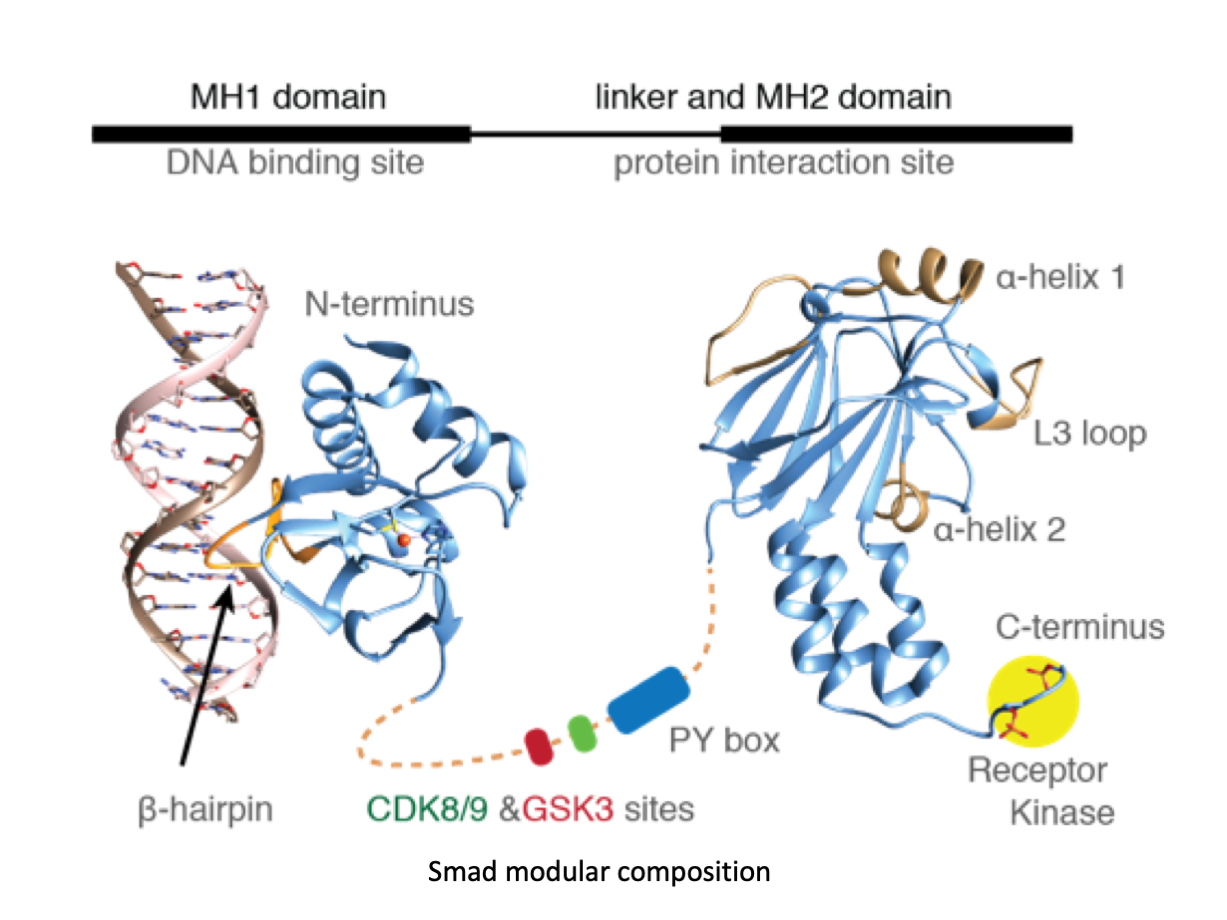
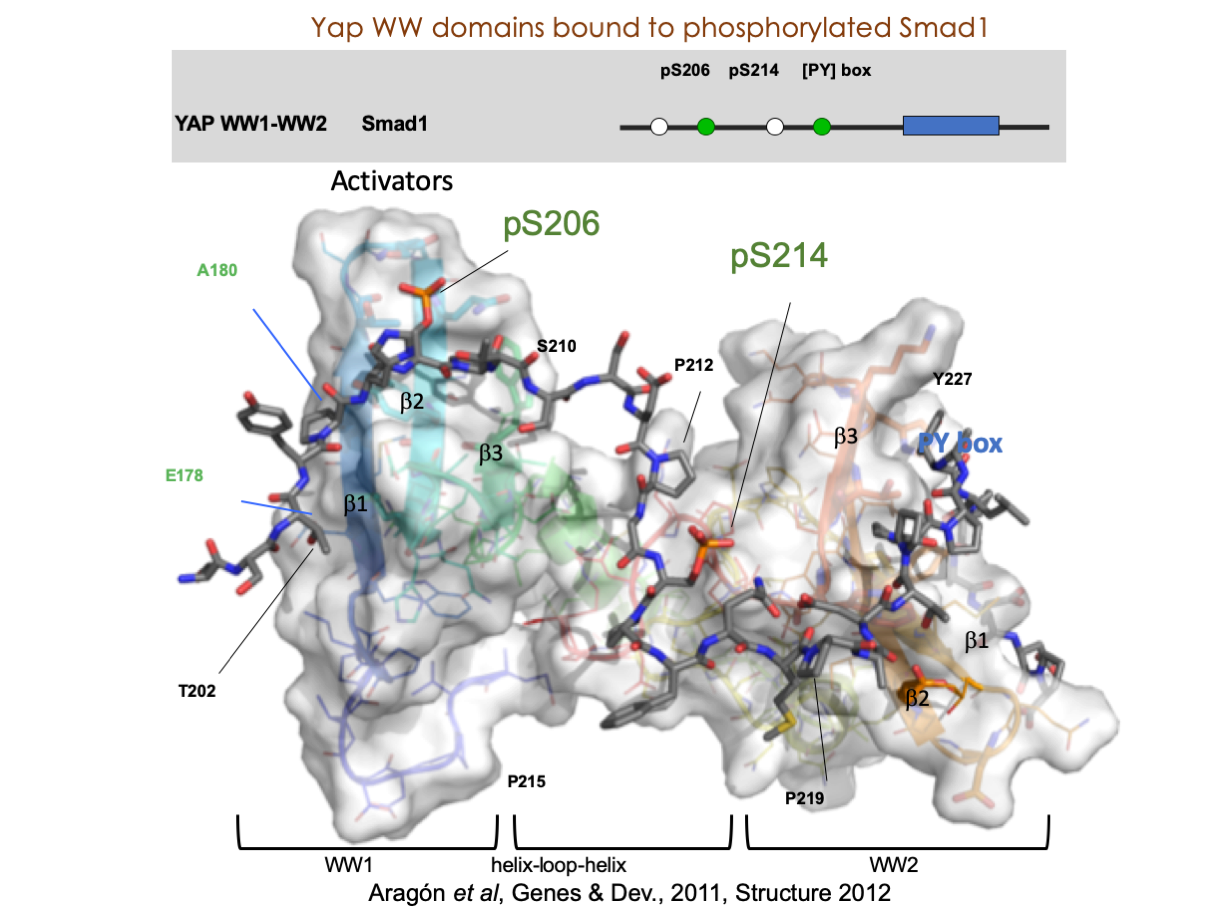
A significant portion of our research is dedicated to elucidating the nuances of TGFβ signaling, with a particular focus on the pivotal roles played by SMAD transcription factors and their associated cofactors. TGFβ signaling is integral to early embryogenesis and developmental processes. Remarkably, numerous malignancies employ analogous strategies to acquire aggressive attributes, thus enhancing their invasive potential and metastatic propensities. Dysregulated TGF-β signaling is implicated in other pathologies like inflammation, fibrosis, and rare diseases as the Myhre syndrome and the Hereditary hemorrhagic telangiectasia.
Since 2008, our fruitful collaboration with Dr. Joan Massagué’s group at the Memorial Sloan Kettering Cancer Center in New York, USA, has been concentrated on various facets of TGFβ signaling and SMAD functionality.
Moreover, in close collaboration with dedicated gynecologic and oncology medical professionals at Sant Pau Hospital in Barcelona, led by Ramon Rovira and colleagues, along with researchers at its affiliated research institute, we are diligently working to identify innovative diagnostic tools and predictive models for assessing the likelihood of relapse and metastasis in cases of Endometrial cancers.